Number
189
September 1999
Mechanical Eyes and Ears
A Fly's Eye View
Sentient Robots
Laser Breakdown Spectrometer
Light Constructions
SPIE Scene
Letters/Editorial
Education
Industry Focus
Product Briefs
Business Briefs
International Technical Groups
An interview with Sergey Mirov and Bob Pitt, Univ. of Alabama; Birmingham, AL
Novel laser breakdown spectrometer provides multi-element analysis for environmental monitoring
What is laser-induced breakdown spectroscopy?
Pitt: Laser-induced breakdown spectroscopy (LIBS) is a simple, rapid, real-time analytical technique based on the analysis of the spectral emission from laser-induced sparks or plasmas (Figure 1). Pulsed laser radiation is focused to a small spot on a sample material. When power densities exceed hundreds of MW/cm2, a high-temperature, high electron density laser spark or microplasma is formed. The temperature of this plasma, initially, is very hot: 104 to 107 deg. C. At such a high temperature, any sample material is broken down, vaporized, and ionized.
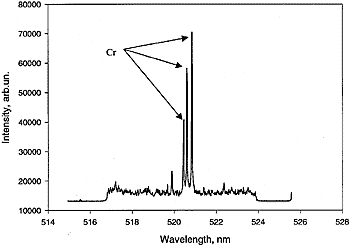
Figure 1. (a) LIBS spectrum of Cr-contaminated (10-ppm) water sample. Three characteristic Cr atomic lines -- 520.844, 520.604, and 520.452 nm-are nicely resolved.
Figure 1. (b) LIBS spectrum of Cu (10-ppm)-contaminated water sample. Atomic lines 324.754 and 327.396 nm are characteristic "fingerprints" for Cu atomic fluorescence. |
In the early stages of plasma thermalization, as electrons interact and recombine with ions, energy will be released over a broad spectral range, from soft x rays through the visible to the near IR. These early stages of plasma glow result in an intense continuum emission that can be gated off by a detection system. After suitable time delays, the plasma cools down to the point when neutral atoms in excited states are formed. Time-resolved spectral analysis of these atomic emissions yield a fingerprint of the atomic species present in the sample (Figure 2).
Figure 2. (a) Time-resolved copper plasma emission generated by the Nd:YAG laser (1024 nm) measured at 0.9, 1.3, 1.7, and 3.1 s after the laser pulse for gating off the continuum emission. Atomic lines 510.554 and 515.324 nm are characteristic "fingerprints" for Cu atomic fluorescence.
Figure 2. (b) Time-integrated spatially resolved LIBS spectrum of Cu (10-ppm)-contaminated water sample generated by the Nd:YAG laser and measured at distances of 5, 3, 2, 1, 0, and -1 mm (which is mainly in the laser spark or microplasma region) from the continuous flowing water jet. |
The greatest advantage of LIBS is the simplicity of experimental arrangement, which makes it cheap. It provides simultaneous multi-element analysis, with no sample preparation. Application of optical fiber techniques to this method enables the construction of a simple and compact device for real time remote sensing of solid, liquid, and gaseous samples.
Are there shortcomings to LIBS?
Mirov: LIBS has several limitations, such as self-absorption of emission, line broadening, and continuum background. These limitations may be minimized by the optimal time gating. Another limitation for quantitative determination of absolute concentrations is related to the relatively low precision (~5 percent) and relatively high limit of detection (up to 100 ppb). If you would like to have a detection limit, say, of subparts per trillion, then definitely, you have to use another method -- laser atomic fluorescence (LAF) spectroscopy.
LAF is based on the absorption of resonant laser radiation by the atomized analyte atom and the detection of the subsequently emitted fluorescence radiation. This technique requires utilization of expensive and sophisticated frequency-tunable lasers with narrow bandwidth to make this light-atom interaction extremely selective. It has the ability to detect as low as subparts per trillion. However, if you are interested in a detection limit, say, of some parts per million, well, definitely, laser breakdown spectroscopy is your choice.
You said that LIBS is a low-precision technique, ~5 percent. If so, why use it? Or how do you overcome such low precision?
Mirov: LIBS is practical for situations that don't require very good precision, but do require very fast, real-time measurements with little or no sample preparation. The niche for this application is significant.
Describe your LIBS system design.
Mirov: The overall system design is shown in Figure 3. It is based on a 0.75-m spectrometer ARC-750 (Acton Research Corp.), intensified thermoelectric-cooled 256 X 1024 CCD camera (Princeton Instruments), a probe with fiber optic guide for signal transportation, and an atomizer based on an Nd:YAG laser (50 mJ, 1.064 µm, 5-ns pulse duration, and 10-Hz pulse repetition frequency) or an alexandrite laser (80 mJ, 0.740 µm, 40 ns, and 20 Hz prf) that generates plasma in the water jet of a continuously circulating sample.
Figure 3. LIBS System Design. The LIBS system consists of four principal components: (1) laser breakdown atomizing module, which includes the laser and nebulizer, (2) the collection optics, which includes the elliptical mirror, (3) the spectrally selective detection system (spectrograph-gated CCD detector combination), and (4) the data acquisition system, which is a notebook computer. |
In order to atomize the sample in the plasma plume, we use two different methods for its delivery. In the first one, the liquid solution containing the atoms to be investigated is sucked into the chamber of the nebulizer, which is a device used to convert a liquid sample into an aerosol. The aerosol -- a mixture of droplets of liquid sample and inert argon gas -- then passes through the nozzle and enters the plasma plume, which is generated by the laser spark in argon. The second approach is based on directly generating plasma in the water jet of the continuously circulating sample. Once in the plasma plume, the impurities are atomized and ionized and emit fluorescence that is collected by an elliptical mirror, directed through the fiber bundle to the slit of the spectrograph, and subsequently measured in real time with the multichannel detection system.
Since the attainable signal is proportional to the fluorescence collection efficiency, it is important to optimize the collection optics. Our collection system uses an elliptical mirror. The laser spark is generated near one of the focal points, and the polished end of the multifiber bundle is placed at the second focal point. The elliptical mirror focuses images of atomic fluorescence into the polished end of the fiber bundle, which transmits the collected fluorescence to the entrance slit of the spectrograph. The exit end of the fiber bundle is arranged in a rectangular shape (4 X 0.1mm) to match it to the entrance slit of the spectrograph.
In our experiments a TE-cooled 256 X 1024 CCD camera with a fiber-coupled intensifier was used for time-gated detection of the fluorescence signal under pulsed laser excitation. Under normal conditions, the intensifier is "closed" and no photons reach the CCD detector. The pulse generator produces a short (5 to 200 ns), high-voltage (200-V) pulse to gate the detector for a 30-µs exposure, 5-µs after the laser pulse, allowing the background continuum to decay. A single-grating monochromator with aperture ratio f/10 and focal length 750 mm was operated as a spectrograph. A multichannel CCD detector was mounted at one of the output ports of the spectrometer. The spectral resolution of this system was estimated to be less than 0.04 nm at 633 nm for a 1200-groove/mm grating. In our experiments, we used 2400- and 1200-g/mm gratings for UV and UV-VIS measurements, respectively.
The resolution of the spectral instrument is approximately equal to RLD times the slit width, where RLD stands for reciprocal linear dispersion and is a feature of a particular device. We cannot change it since it depends on the type of grating we use and the focal length of the spectrometer. The slit width, on the other hand, can be easily changed from 10 µm to 3 µm. When we increase the slit width, we decrease the spectral resolution but increase the signal. So, there is a trade-off between the intensity of the detected signal and spectral resolution of the instrument. Our measurement was with a slit width of 10 µm.
Every heavy metal atom features specific spectral signatures. For example, if we see lines around 324 nm, we know this line belongs to a copper atom. When we see lines in some other spectral region, we can open any reference book of atomic lines, and say this is, for example, an emission line of lead, mercury, chromium, and so on. We immediately understand the chemical composition of a sample by analyzing their spectral lines on the screen of our monitor. So, it is real-time spectroscopy.
Sergey Mirov at work. |
How would you use this for biological warfare agents such as anthrax? Is it different than heavy metals?
Mirov: Actually, there is no difference because we tie the detection of anthrax spores to the detection of heavy metal atoms that can be selectively attached to the spores. The method is pretty straightforward and is completely analogous to fluorescently labeled antibody biosensing. Antibodies are substituted by special ligands (proteins) featuring selective affinity to anthrax spores, and instead of dye labeling, one may use heavy metal microspheres. The size of the metal microsphere (4 µm) is chosen to match the average size of the anthrax spores. So, on average, each spore will be bonded to one metal microsphere. Simple calculations show that each 4-µm metal microsphere contains about 1012 atoms of metal. A 100 ppb level of detectability of the method corresponds to ~1015 atoms/cm3 or 1000 spores per 1 cm3 of water. For the 10 m3 of the ambient air intake volume forced through 10 cm3 of water in the accumulation chamber, the final detectability of the proposed system turns out to be ~1000 spores/m3. It can be further significantly improved by laser breakdown atomization with a resonant laser excitation of the metal atoms.
Anything else?
Pitt: We managed to obtain a very good level of detectability, 100 parts per billion. It is very good for laser breakdown spectroscopy. We will do even better when we have much better collection optics to capture more fluorescence. Also, in our system, we have a very nice method of capturing parts of the sample. We have a special water jet that provides continuous circulation of the sample, much like the technology used in tunable dye lasers where you pump the dye through the appropriate jet. So, we don't lose the sample and we can use very small quantities of the sample for making measurements. We directly atomize this stream of water.
Do you think this sort of system would be commercially viable? Why?
Mirov: Yes, because LIBS is a simple, rapid, in-situ method of considerable importance for analytical applications.
 |
Sergey Mirov is a Professor of Physics at the Univ. of Alabama at Birmingham. He is engaged in laser, laser atomic, and Raman spectroscopy, the physics of laser media, and laser applications in environmental science and biomedicine. He is a member of both OSA and SPIE. |
 |
Bob Pitt is a professor of Civil and Environmental Engineering at the Univ. of Alabama at Birmingham. His main area of research involves toxicant investigations of urban stormwater, especially methods that can be used in the field with minimal sample preparation. |
Mirov and Pitt were interviewed by Frederick Su.
